前列腺癌,美国在研的481项基金简述(2024)
2024-09-18 Hanson临床科研 Hanson临床科研
介绍前列腺癌特点及未解决临床问题,梳理美国 NIH 资助的前列腺癌在研项目,包括获资助者、研究热点及课题摘要,为选题提供启发。
前列腺癌(Prostate Cancer)是全球男性中最常见的癌症类型之一,它起源于前列腺腺体的细胞,前列腺是男性生殖系统的一部分,位于膀胱下方,尿道穿过前列腺。这种癌症的特点和治疗响应在不同患者间表现出显著差异。
尽管近年来在前列腺癌的诊断和治疗方面取得了显著进展,但仍存在一些重要而未解决的临床问题:
-
早期诊断的挑战:黑色素瘤在早期往往缺乏明显的临床症状,导致许多患者在确诊时已经处于疾病晚期。如何提高早期诊断的准确性和效率,是当前面临的一个重要问题。
-
筛查争议:关于前列腺癌的筛查,特别是前列腺特异性抗原(PSA)测试的使用存在争议。虽然PSA测试可以帮助早期发现癌症,但也可能导致过度诊断和过度治疗,包括对可能永远不会对健康构成威胁的癌症进行治疗。
-
治疗选择:对于局限于前列腺的癌症,治疗选择包括手术、放疗、激素治疗等。然而,确定最适合特定患者的治疗方案仍然具有挑战性,因为治疗选择需要在有效控制疾病和最小化副作用之间进行权衡。
-
晚期前列腺癌治疗:尽管对早期前列腺癌有多种有效的治疗选择,晚期前列腺癌的治疗仍然是一个挑战。晚期前列腺癌对常规激素治疗可能产生抵抗,需要更多的治疗创新。
-
个体化医疗:如何根据个体的遗传背景和病理特征来定制治疗策略,以提高治疗效果和患者生活质量,是当前研究的热点。
这些问题的解决需要进一步的科学研究和临床试验,以优化筛查策略,精确治疗,并改善患者的生存率和生活质量。通过更深入地了解前列腺癌的分子机制,未来可能开发出更有效的治疗方法。
我们仅对美国国立卫生研究院(NIH)资助的在研前列腺癌相关项目进行梳理,希望给同仁们的选题思路提供一点启发。
2024年,以“Prostate Cancer”为检索词、在题目中进行检索,美国NIH针对前列腺癌的在研有481项。
一,谁获得了这些研究?
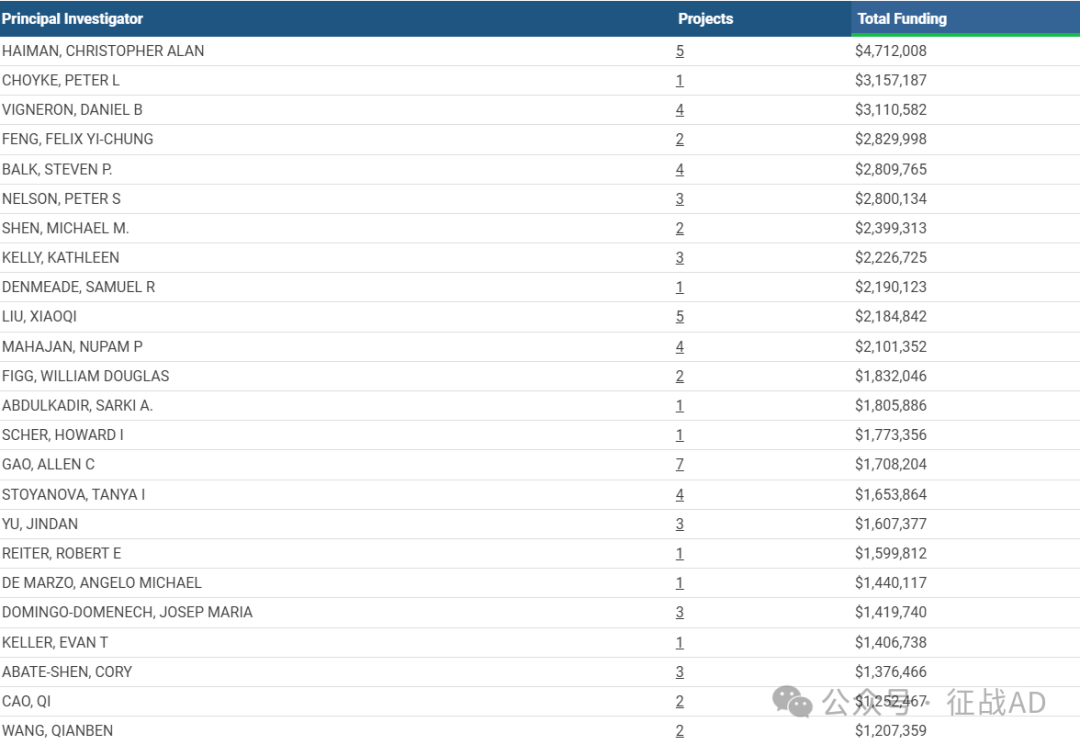
1,在研前列腺癌基金最多的PI
-
UNIVERSITY OF SOUTHERN CALIFORNIA 的 HAIMAN, CHRISTOPHER ALAN
-
BASIC SCIENCES 的 CHOYKE, PETER L
-
UNIVERSITY OF CALIFORNIA, SAN FRANCISCO 的 VIGNERON, DANIEL B
-
UNIVERSITY OF CALIFORNIA, SAN FRANCISCO 的 FENG, FELIX YI-CHUNG
-
BETH ISRAEL DEACONESS MEDICAL CENTER 的 BALK, STEVEN P.
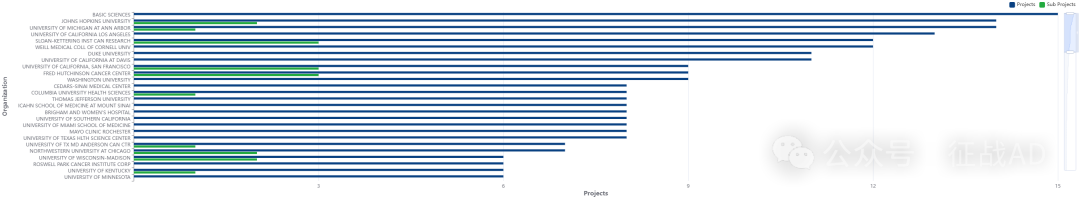
2,前列腺癌基金最多的研究机构
-
基础科学
-
约翰霍普金斯大学
-
加州大学旧金山分校
-
密歇根大学安娜堡分校
-
南加州大学等
二,前列腺癌研究热点是什么?
前列腺癌研究领域总览(根据关键词)
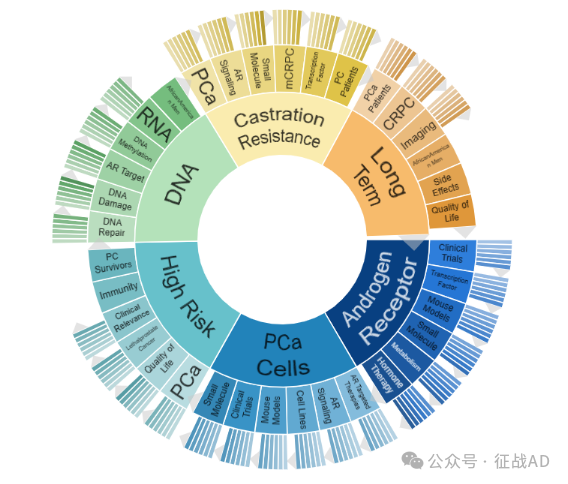
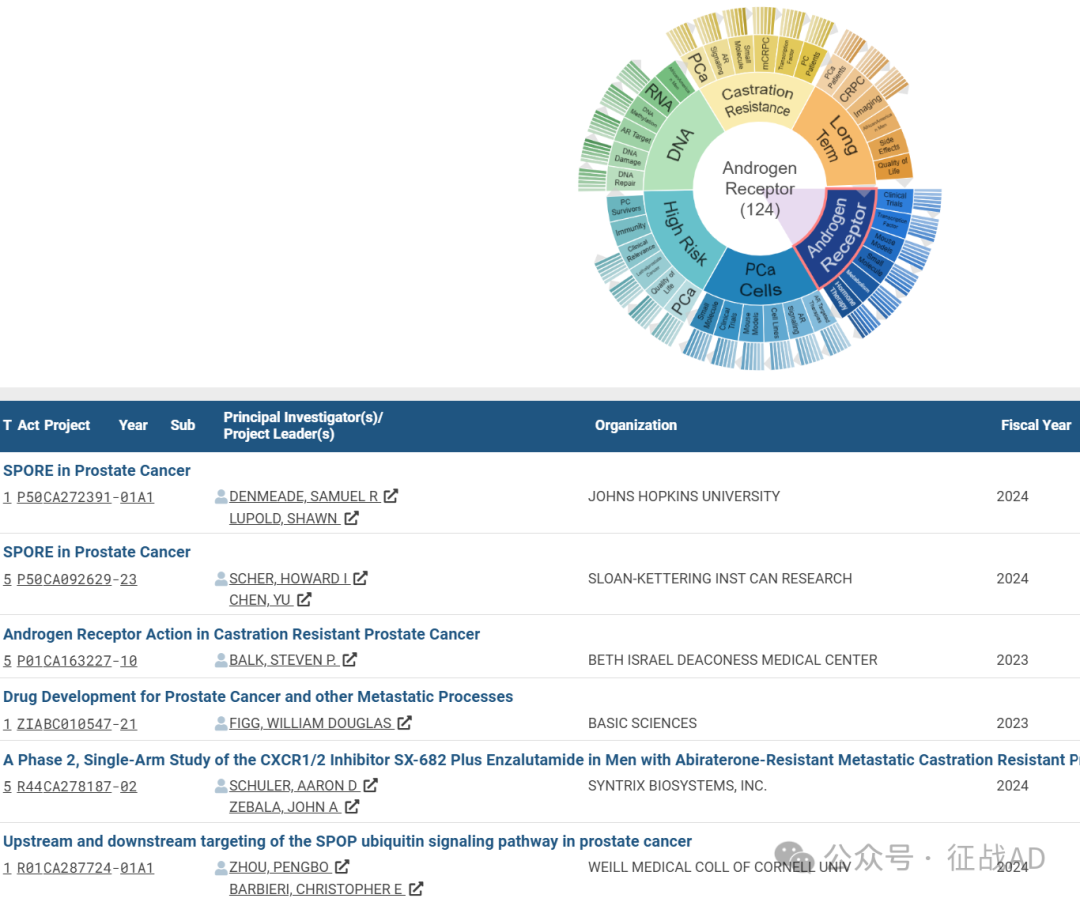
A,关于雄激素受体(Androgen Receptor)的研究项目最多
有 124 项在研基金涉及到了雄激素受体,关注最多的方面包括临床研究(Clinical Trials)、转录因子(Transcription Factor)、小鼠模型(Mouse Models)、小分子(Small Molecule)、代谢(Metabolism)、激素治疗(Hormone Therapy)等方面研究。
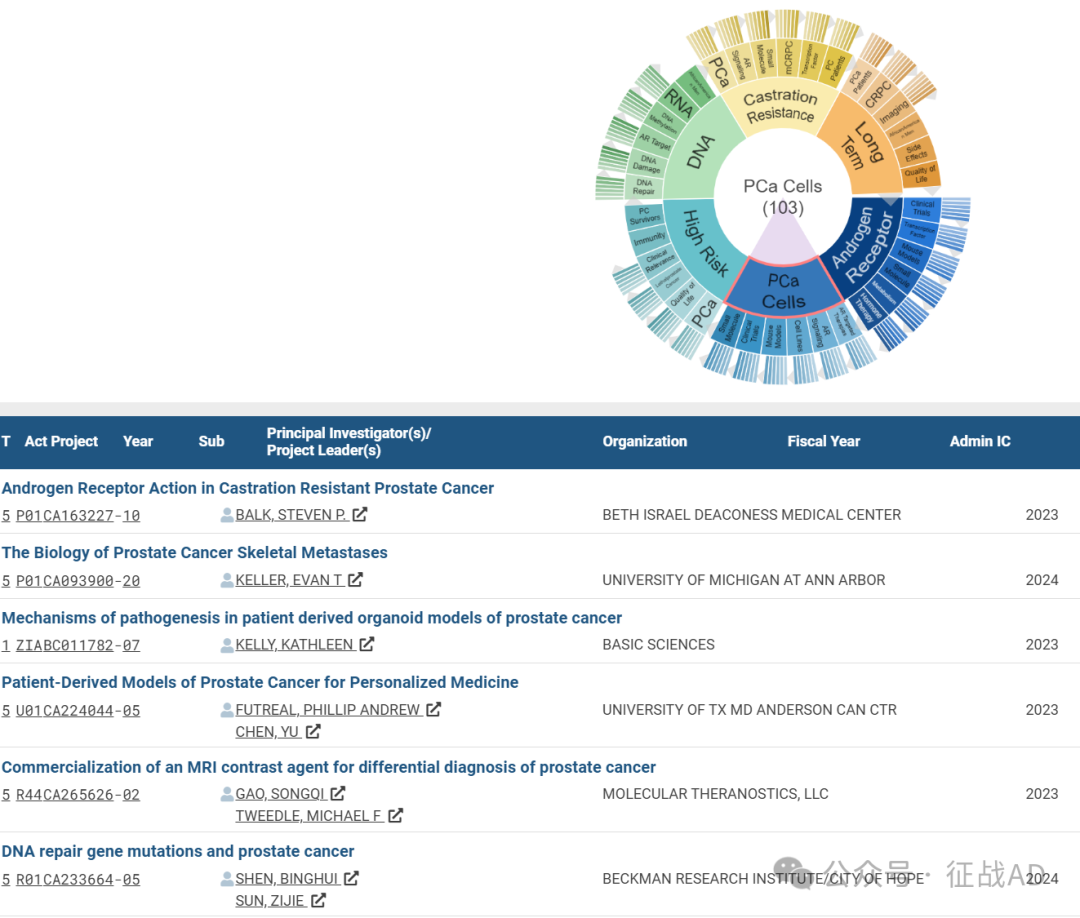
B,前列腺癌细胞(PCa Cells)的研究
有 103 项研究涉及到前列腺癌细胞,研究领域主要涉及AR靶向治疗(AR Targeted Therapies)、AR信号(AR Signaling)、细胞系(Cell Lines)、小鼠模型(Mouse Models)、临床研究(Clinical Trials)、小分子(Small Molecule)等方面研究。
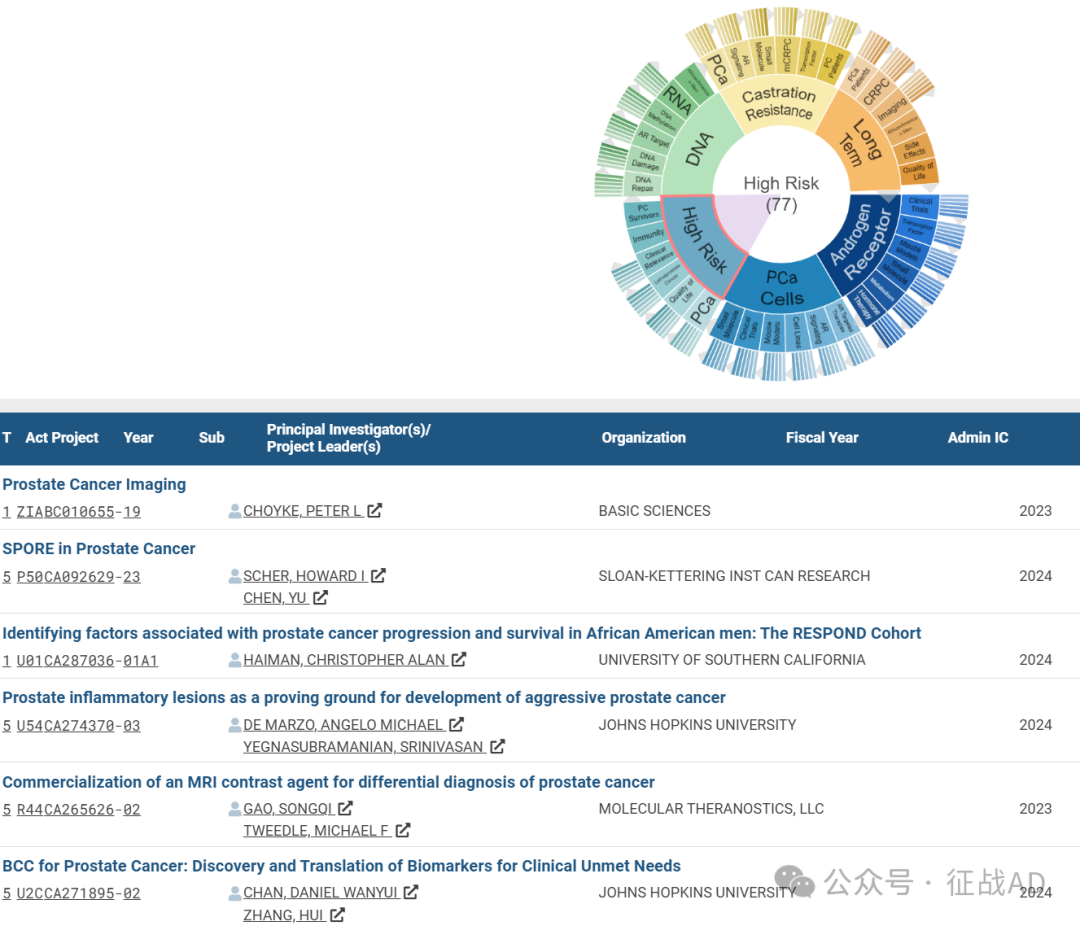
C,高风险(High Risk)
有 77 项研究涉及到高风险,涉及的关键词包括PCa、生活质量(Quality of Life)、致死性前列腺癌(Lethalprostate Cancer)、临床相关性(Clinical Relevance)、免疫(Immunity)、前列腺癌幸存者(PC Survivors)等。
其他前列腺癌研究大的方向也包括DNA、去势抵抗(Castration Resistance)、长期(Long Term)等。
三,借鉴与突破
我们也分享在前列腺癌领域的几项课题摘要,希望对同仁们有所启发。
A,The Biology of Prostate Cancer Skeletal Metastases
The central theme of this Program is that there is crosstalk between PCa cells and the bone microenvironment that fosters the development and progression of PCa metastasis. This crosstalk promotes the ability of PCa cells to alter the bone microenvironment and render it fertile for tumor growth and chemotherapeutic resistance. To expand on this theme the Program encompasses closely interrelated hypotheses of four scientific projects supported by three cores. Project 1 explores the novel finding that chemotherapy induces fusion of PCa cells to form multinuclear polyploid giant cancer cells (PGCCs) that confer chemoresistance in the bone microenvironment; Project 2 examines the exciting idea that abscisic acid (ABA) induces PCa cells to adopt a phenotype capable of existing in a dormant and chemoresistant state, with the capacity for long-term survival and potential to develop into overt bone metastases; Project 3 explores the surprising role that osteocytes (OCys) play in promoting PCa bone metastasis through activation of a novel growth differentiation factor-15 (GDF15) receptor, GDFN family receptor alpha-like precursor (GFRAL), that subsequently promotes PCa metastatic invasion and growth; Project 4 investigates the novel hypothesis that macrophage efferocytosis (engulfment) of apoptotic PCa cells induces immunosuppressive signaling in the bone microenvironment that subsequently enhances metastatic growth.
These projects will be supported by three integral cores: Core A (Administration) that will coordinate reporting, evaluation of progress, advisory board activities, facilitate interactions among the projects and provide biostatistical support; Core B (Animal) will provide mouse models and imaging and assistance with their use and Core C (Bone) will provide expertise with bone histology processing, interpretation, and procurement of human blood and bone marrow samples.
B, Prostate inflammatory lesions as a proving ground for development of aggressive prostate cancer
Epidemiological and pathological studies have implicated lifestyle, microbial, and environmental factors in prostate cancer etiology/risk. A potential link between these factors and prostate carcinogenesis is the presence of chronic inflammation associated with atrophy (PIA) in prostates of aging men. Yet, there is a paradox surrounding the role of the immune response in prostate cancer: “the inflammation paradox”. On one hand, inflammation may be a driver of carcinogenesis. On the other, the immune system is known to seek and destroy cancer cells. The majority prostate cancer lesions are “immune deserts”, and ICIs are ineffective in most cases. Why is there an evidently strong immune reaction in non- neoplastic regions in PIA, but a lack of a robust immune response in most prostate cancers? We hypothesize that chronic inflammation in PIA represents evidence of an innate immune response that drives carcinogenesis. However, in this inflammatory “proving ground”, only cells that can epigenetically switch off this response can emerge to become aggressive neoplastic precursors. We hypothesize that the paucity of immune infiltrates and lack of PD-L1, is evidence that prostate cancer cells develop a number of different mechanisms that evade anti-tumor adaptive immunity. We postulate that additional cell non- autonomous immune suppressive mechanisms enable disease progression.
We propose 3 synergistic Research Projects (2 basic,1 translational) to mechanistically test key questions stemming from our “proving ground” hypothesis.
In Proj 1 (Basic Science) we hypothesize that the STING induction in PIA drives acute and chronic inflammation, leading to cell injury/cell death and proliferation. Second, in a subset of PIA cells, epigenetic silencing of STING dampens of the immune response, allowing them to emerge as overt pre- neoplastic cells. We will test this in animal models and in translational studies employing annotated and molecularly characterized prostatectomies. The combination of PTEN loss and MYC copy number gain is an independent predictor of poor outcome in prostate cancer. We hypothesize that the combination of MYC and PTEN stimulates a cell non-autonomous immune evasion mechanism induced by the recruitment of immuno- suppressive myeloid cells, and fibroblast activation protein (FAP)-positive fibroblasts.
Proj 2 (Basic Science) will test these hypotheses in animal models and in human tissues. Recently introduced imaging technologies have raised the hypothesis that PET/CT imaging results may be able to predict molecular and tumoral micro- environmental characteristics of aggressive prostate cancer. PET imaging for PSMA using PyL PET/CT has been FDA approved for imaging high risk men prior to prostatectomy.
In Proj 3 (Translational) we employ PET/CT scanning for PSMA and combine this with mpMRI to address these hypotheses. Also in Proj 3 we will apply newly developed/developing PET imaging agents to non-invasively and longitudinally study the extent of M2 macrophages and cancer associated fibroblasts in our mouse prostate progression cancer models.
作者:Hanson临床科研
版权声明:
本网站所有注明“来源:梅斯医学”或“来源:MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明“来源:梅斯医学”。其它来源的文章系转载文章,本网所有转载文章系出于传递更多信息之目的,转载内容不代表本站立场。不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言











#前列腺癌# #研究热点#
0