The Lancet:达珠单抗HYP能有效治疗复发-缓解型多发性硬化
2013-04-17 The Lancet dxy
达珠单抗HYP治疗复发-缓解型多发性硬化患者的疗效 达珠单抗是一种人源性的单克隆抗体,其主要通过阻滞白细胞介素-2受体α亚单位(CD25)来介导白细胞介素-2信号传导过程。来自德国St Josef医院的Ralf Gold等为了评估对于复发-缓解型多发性硬化患者而言,达珠单抗HYP作为单药治疗(疗程1年)的有效性而设计了相关的研究,他们的研究结果发表在The Lancet 4月最新的在线期刊上。本
达珠单抗是一种人源性的单克隆抗体,其主要通过阻滞白细胞介素-2受体α亚单位(CD25)来介导白细胞介素-2信号传导过程。来自德国St Josef医院的Ralf Gold等为了评估对于复发-缓解型多发性硬化患者而言,达珠单抗HYP作为单药治疗(疗程1年)的有效性而设计了相关的研究,他们的研究结果发表在The Lancet 4月最新的在线期刊上。
本研究为随机、双盲、安慰剂对照研究,研究在捷克共和国、德国、匈牙利、印度、波兰、俄国、乌克兰、土耳其和英国的76个中心内进行,研究进行的时间为2008年2月15日至2010年5月14日。本研究所纳入的受试者为年龄在18岁至55岁、罹患复发-缓解型多发性硬化的患者。研究者采用中央交互式语音应答系统,将符合入组标准的患者按照1:1:1的比例随机分为3组,分别皮下注射达珠单抗HYP 150mg、300mg或安慰剂,每4周注射一次,疗程为52周。在本研究中,患者和研究者都不知道患者所接受的具体治疗方案,但是在每个中心内负责准备试验用药的药剂师知晓患者所接受的治疗方案,不过,药师与患者没有直接接触。本研究的主要终点事件为年化复发率。研究者采用意向治疗分析法对研究结果进行分析。本研究在ClinicalTrials.gov注册,注册号为NCT00390221。
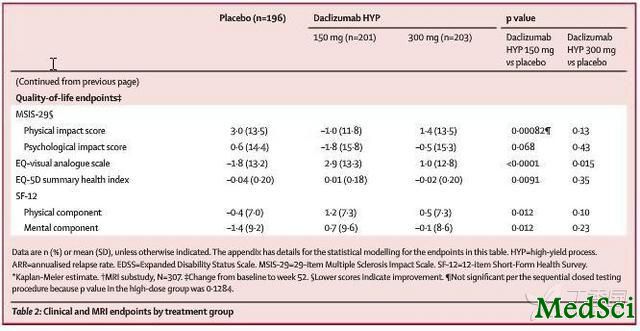
在本研究中,安慰剂组的患者为204人、达珠单抗HYP 150mg组的患者为208人,达珠单抗HYP 300mg组的患者为209人,完整的完成了52周随访的患者在三组中分别为188人(92%)、192人(92%)和197人(94%)。与安慰剂组的患者相比,达珠单抗HYP 150mg组和300mg组的患者的年化复发率更低,在150mg组为0.21,在300mg组为0.23,而在安慰剂对照组则为0.46,差异具有显著统计学意义。此外,与安慰剂对照组的患者相比,达珠单抗HYP 150mg组和300mg组的患者中处于无复发状态的人更多,150mg组为81%,300mg组为80%,而安慰剂对照组则为64%,差异同样具有显著统计学意义。在安慰剂对照组、150mg组和300mg组出现除多发性硬化复发之外的严重不良反应事件的患者分别为12人(6%)、15人(7%)和19人(9%)。在达珠单抗HYP 150mg组中有一名患者出现了严重的皮疹,在皮疹的恢复过程中其因腰肌脓肿而死亡。
本研究结果指出,在一年的治疗期中,每4周进行1次皮下注射达珠单抗HYP能有效治疗多发性硬化。本研究结果指出,对于复发-缓解型多发性硬化患者而言,达珠单抗HYP 是其额外治疗的选择之一。
与多发性硬化相关的拓展阅读:
- The Lancet:达珠单抗HYP能有效治疗复发-缓解型多发性硬化
- FDA批准Tecfidera治疗多发性硬化症
- FDA批准富马酸二甲酯治疗多发性硬化
- ANN NEUROL :多发性硬化联合治疗与单一疗法的比较
- PLOS ONE:抗癌药伊马替尼可缓解多发性硬化症
- Neurology:女孩肥胖增加多发性硬化发生风险 更多信息请点击:有关多发性硬化更多资讯

Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECT): a randomised, double-blind, placebo-controlled trial
Background
Daclizumab, a humanised monoclonal antibody, modulates interleukin-2 signalling by blocking the α subunit (CD25) of the interleukin-2 receptor. We assessed whether daclizumab high-yield process (HYP) would be effective when given as monotherapy for a 1 year treatment period in patients with relapsing-remitting multiple sclerosis.
Methods
We did a randomised, double-blind, placebo-controlled trial at 76 centres in the Czech Republic, Germany, Hungary, India, Poland, Russia, Ukraine, Turkey, and the UK between Feb 15, 2008, and May 14, 2010. Patients aged 18—55 years with relapsing-remitting multiple sclerosis were randomly assigned (1:1:1), via a central interactive voice response system, to subcutaneous injections of daclizumab HYP 150 mg or 300 mg, or placebo, every 4 weeks for 52 weeks. Patients and study personnel were masked to treatment assignment, except for the site pharmacist who prepared the study drug for injection, but had no interaction with the patient. The primary endpoint was annualised relapse rate. Analysis was by intention to treat. The trial is registered with ClinicalTrials.gov, number NCT00390221.
Findings
204 patients were assigned to receive placebo, 208 to daclizumab HYP 150 mg, and 209 to daclizumab HYP 300 mg, of whom 188 (92%), 192 (92%), and 197 (94%), respectively, completed follow-up to week 52. The annualised relapse rate was lower for patients given daclizumab HYP 150 mg (0·21, 95% CI 0·16—0·29; 54% reduction, 95% CI 33—68%; p<0·0001) or 300 mg (0·23, 0·17—0·31, 50% reduction, 28—65%; p=0·00015) than for those given placebo (0·46, 0·37—0·57). More patients were relapse free in the daclizumab HYP 150 mg (81%) and 300 mg (80%) groups than in the placebo group (64%; p<0·0001 in the 150 mg group and p=0·0003 in the 300 mg group). 12 (6%) patients in the placebo group, 15 (7%) of those in the daclizumab 150 mg group, and 19 (9%) in the 300 mg group had serious adverse events excluding multiple sclerosis relapse. One patient given daclizumab HYP 150 mg who was recovering from a serious rash died because of local complication of a psoas abscess.
Interpretation
Subcutaneous daclizumab HYP administered every 4 weeks led to clinically important effects on multiple sclerosis disease activity during 1 year of treatment. Our findings support the potential for daclizumab HYP to offer an additional treatment option for relapsing-remitting disease.
Funding
Biogen Idec and AbbVie Biotherapeutics Inc.
作者:The Lancet
版权声明:
本网站所有注明“来源:梅斯医学”或“来源:MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明“来源:梅斯医学”。其它来源的文章系转载文章,本网所有转载文章系出于传递更多信息之目的,转载内容不代表本站立场。不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言





#Lancet#
31
#多发性#
32
#有效治疗#
42