NEJM:Nintedanib系统性硬化相关的肺间质疾病的效果
2019-06-17 佚名 SCI天天读
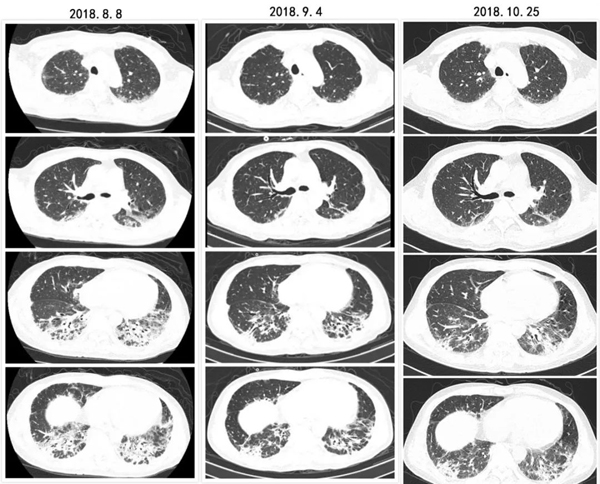
间质性肺疾病(ILD)是系统性硬化症(SS)的常见表现,是导致SS死亡的主要原因。Nintedanib是一种酪氨酸激酶抑制剂(TKI),在SS和ILD的临床前研究中已被证明具有抗纤维化和抗炎作用。
作者:佚名
版权声明:
本网站所有注明“来源:梅斯医学”或“来源:MedSci原创”的文字、图片和音视频资料,版权均属于梅斯医学所有。非经授权,任何媒体、网站或个人不得转载,授权转载时须注明“来源:梅斯医学”。其它来源的文章系转载文章,本网所有转载文章系出于传递更多信息之目的,转载内容不代表本站立场。不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
在此留言





#TED#
43
#系统性#
34
#Nintedanib#
40
#系统性硬化#
40
顶刊就是顶刊,谢谢梅斯带来这么高水平的研究报道,我们科里同事经常看梅斯,分享梅斯上的信息
51